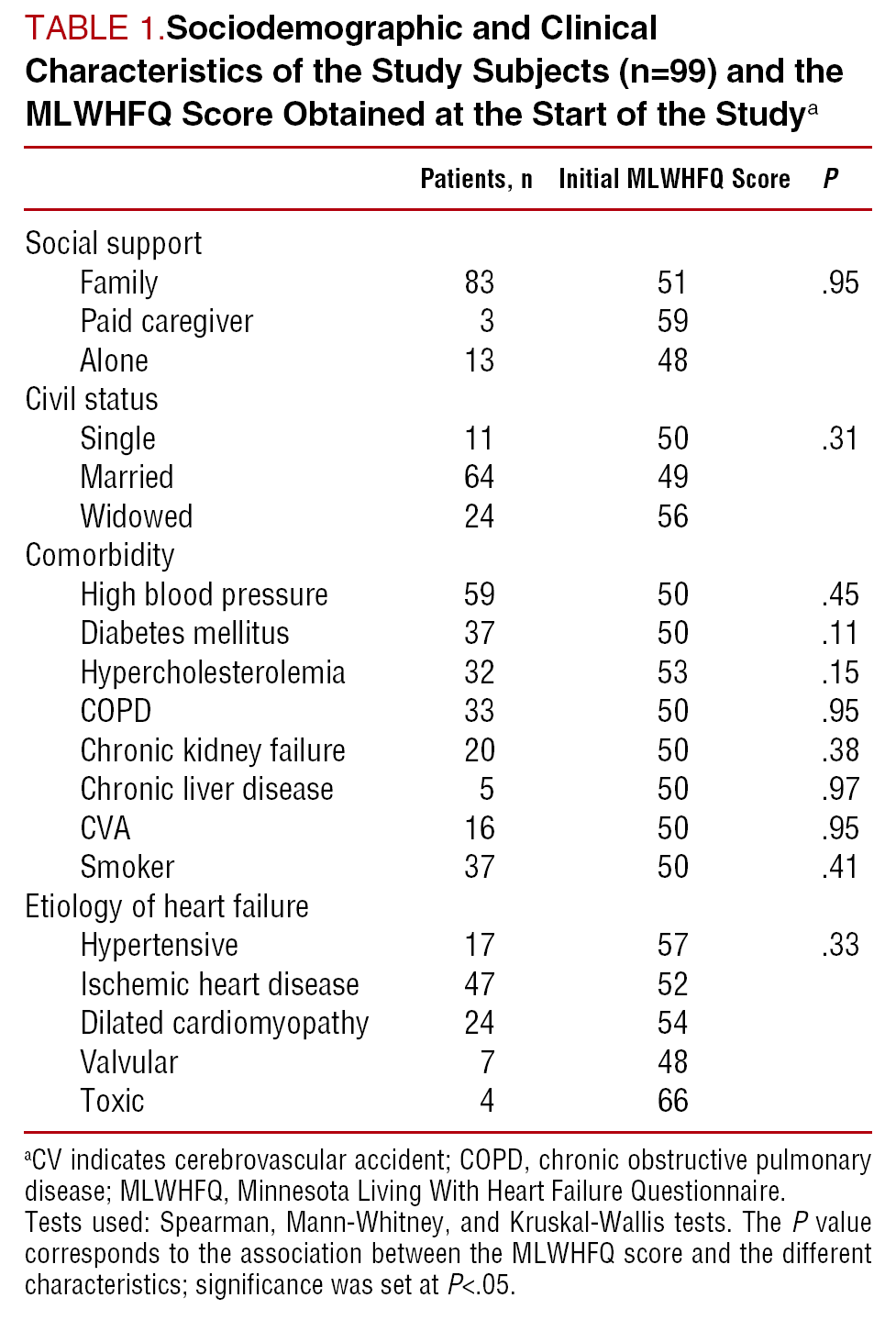
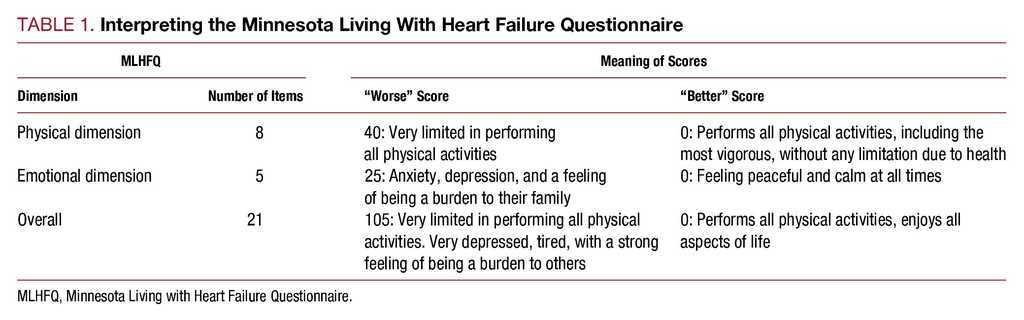
Minnesota living with heart failure questionnaire heart failure health related quality of life factor analysis rasch analysis psychometric properties background heart failure hf is one of the most important health problems in terms of prevalence morbidity mortality and health service use 1.
Minnesota living with heart failure questionnaire pdf.
Rector ts kubo cohn jn.
A qualitative validation of the minnesota living with heart failure questionnaire article pdf available in quality of life research 13 2 417 26 april 2004 with 567 reads how we measure reads.
The minnesota living with heart failure questionnaire mlhfq is one of the most widely used health related quality of life questionnaires for patients with heart failure hf.
It provides scores for two dimensions physical and emotional and a total score.
The university of.
However there are some concerns about its factor structure and alternatives have been proposed some including a third factor.
The united states food drug administration fda has approved the minnesota living with heart failure questionnaire mlhfq as a medical device development tool mddt for use in controlled clinical trials designed to test superiority or non inferiority of medical devices in support of regulatory submissions.
11 10 04 minnesota living with heart failure questionnaire the following questions ask how much your heart failure heart condition affected your life during the past month 4 weeks.
Patients self assessment of their congestive heart failure.
Am heart j 1992 124 1017 1025 pubmed abstract rector ts kubo sh cohn jn.
Half of the 21 item minnesota living with heart failure questionnaire mlhfq response categories are labeled 0 no 1 very little 5 very much and half are not 2 3 and 4.
Content reliability and validity of a new measure the minnesota living with heart failure questionnaire.
The minnesota living with heart failure questionnaire mlhfq is a reliable and valid patient oriented measure of the adverse effects of heart failure on a patient s life.
It affects around 2 to 3 of.
The united states food and drug administration fda has approved the minnesota living with heart failure questionnaire as medical device development tool mddt for use in controlled clinical trials designed to test.